From Molecules to Atmospheres.
How relevant is chemistry to atmospheric systems? How the interaction of molecules among themselves and interaction of molecules with light affects the gas phase surrounding planets? What tools can be used to understand the mechanisms operating in planetary atmospheres?
These are the questions that this laboratory aims to answer.
Research
This laboratory focuses on chemical processes relevant to planetary atmospheres, including those of present-day and Archean Earth, as well as Mars, Venus, and exoplanets. Understanding these chemical processes is not only applicable but essential for studying the chemical composition of these varied atmospheres.
What specific types of research have been conducted recently?
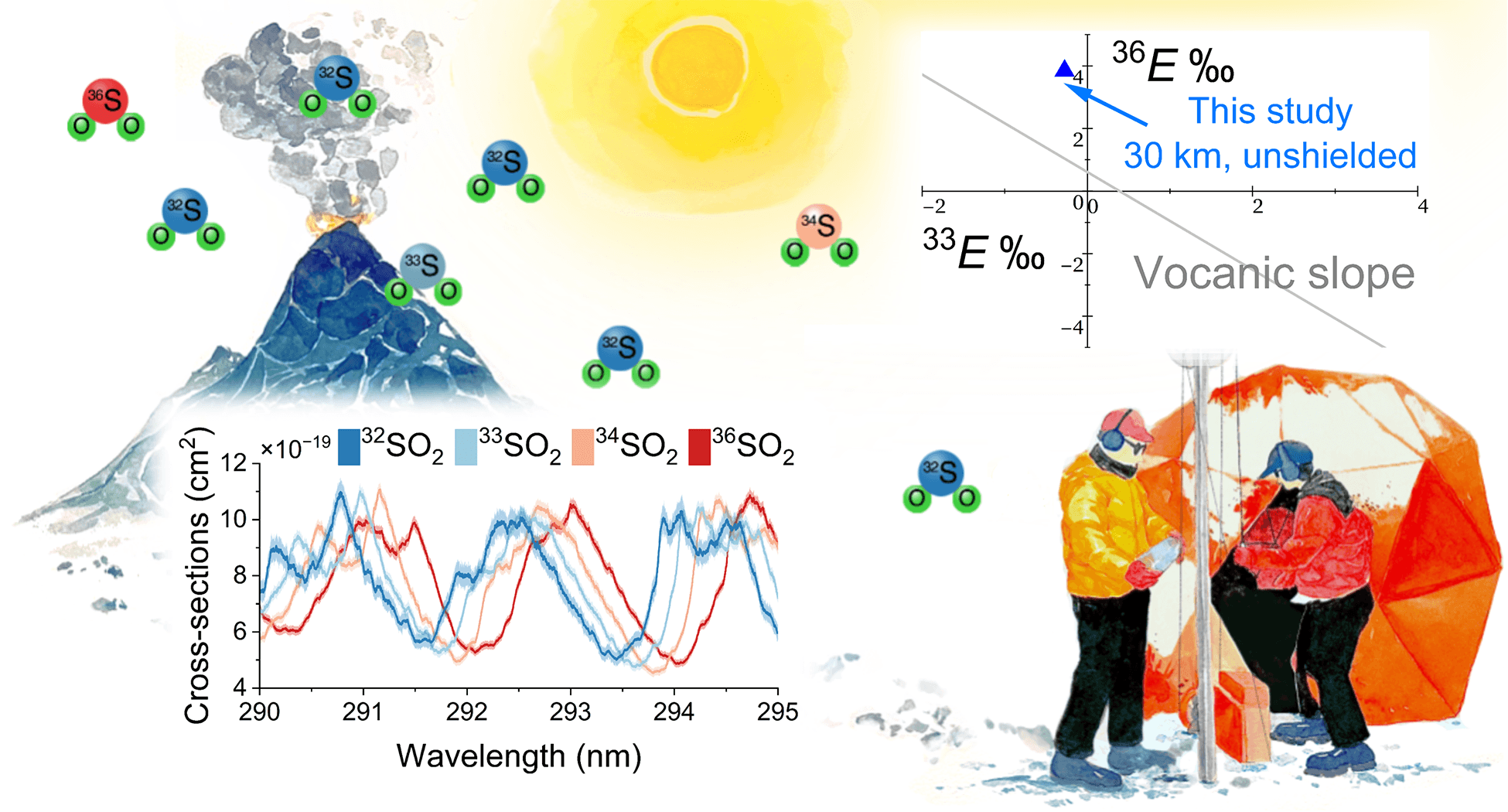
Quantification of Non-Mass-Dependent Sulfur Isotopic Effects in SO₂ Photochemistry
In this research, we use ultraviolet absorption spectroscopy combined with numerical methods for post-experimental data analysis.
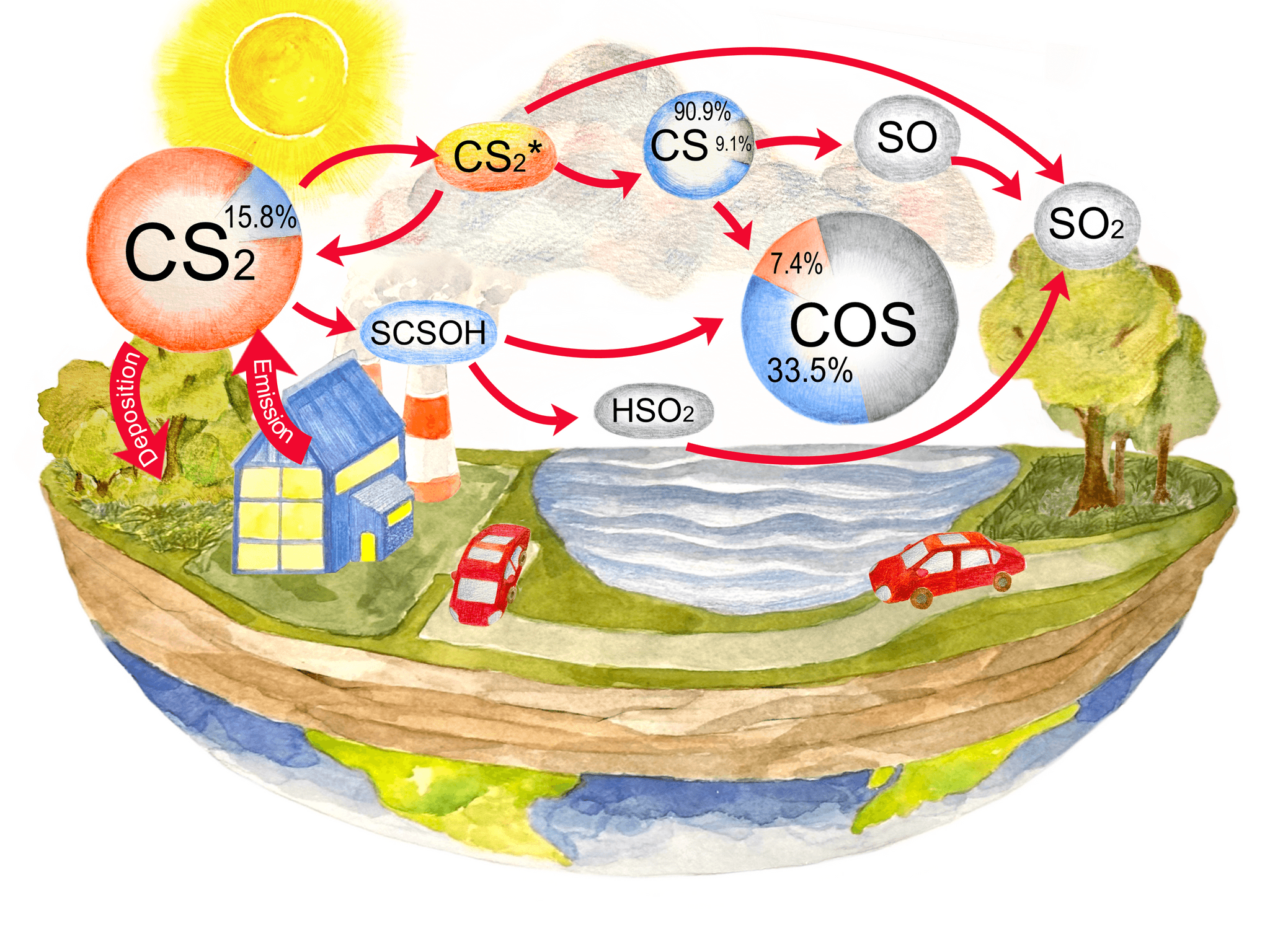
Stratospheric Photochemistry of OCS and H₂SO₄ to Understand the Formation of Stratospheric Sulfur Aerosols
This study involves incorporating the photochemical properties of OCS, H₂SO₄, and related molecules into photochemical models.
Development of Atmospheric Photochemical Models to Account for Light-Induced Isotopic Effects
We develop Python and Fortran codes to numerically quantify isotopic effects in atmospheric processes
Hot topics at the moment
Space and Chemistry
How did the evolution of interstellar clouds to protoplanetary systems reach molecular stability? How did the organic molecules observed in interstellar clouds evolve? What chemical reactions produced the various molecules, in other words, what chemical reaction networks were the products? A chemical reaction network is composed of the rate constants of various reactions, and is the dominant parameter for what can be produced, what cannot be produced, and what can be produced but is easily broken down. In the field of astrochemistry, countless rate constants have been studied for many years, and the accumulated data has been compiled into a database that is currently being introduced into numerical model research.
Before we can calculate the rate constants for photodissociation reactions, we need to determine the UV spectra of the molecules. In other words, in order to study the UV absorption cross-sections and photodissociation mechanisms of various molecules and their isotopes, it is necessary to use chemical models to study the formation of interstellar organic matter and the stability of UV light, but when experimentally determining absorption spectra, it is difficult to reproduce the high vacuum, low temperature conditions of interstellar clouds, and the data obtained from measurements differs greatly from the actual conditions of interstellar clouds. Furthermore, it is necessary to investigate the stability, photodissociation, and isotope enrichment of organic molecules in ice due to ultraviolet energy.
I have been studying isotope effects in absorption UV spectra for many years, and have succeeded in introducing non-adiabatic transitions and cross-effects into the calculation method, achieving a high level of reproducibility between calculated and measured values. The figure shows a comparison between experimental and calculated values. In addition, the ability to calculate molecular dynamics that takes into account excited states and non-adiabatic transitions has made it possible to study photodissociation reactions in clusters of tens of atoms. Now that the reliability of first-principles calculations has been established, we would like to expand our research area to conditions that cannot be measured by experiment.